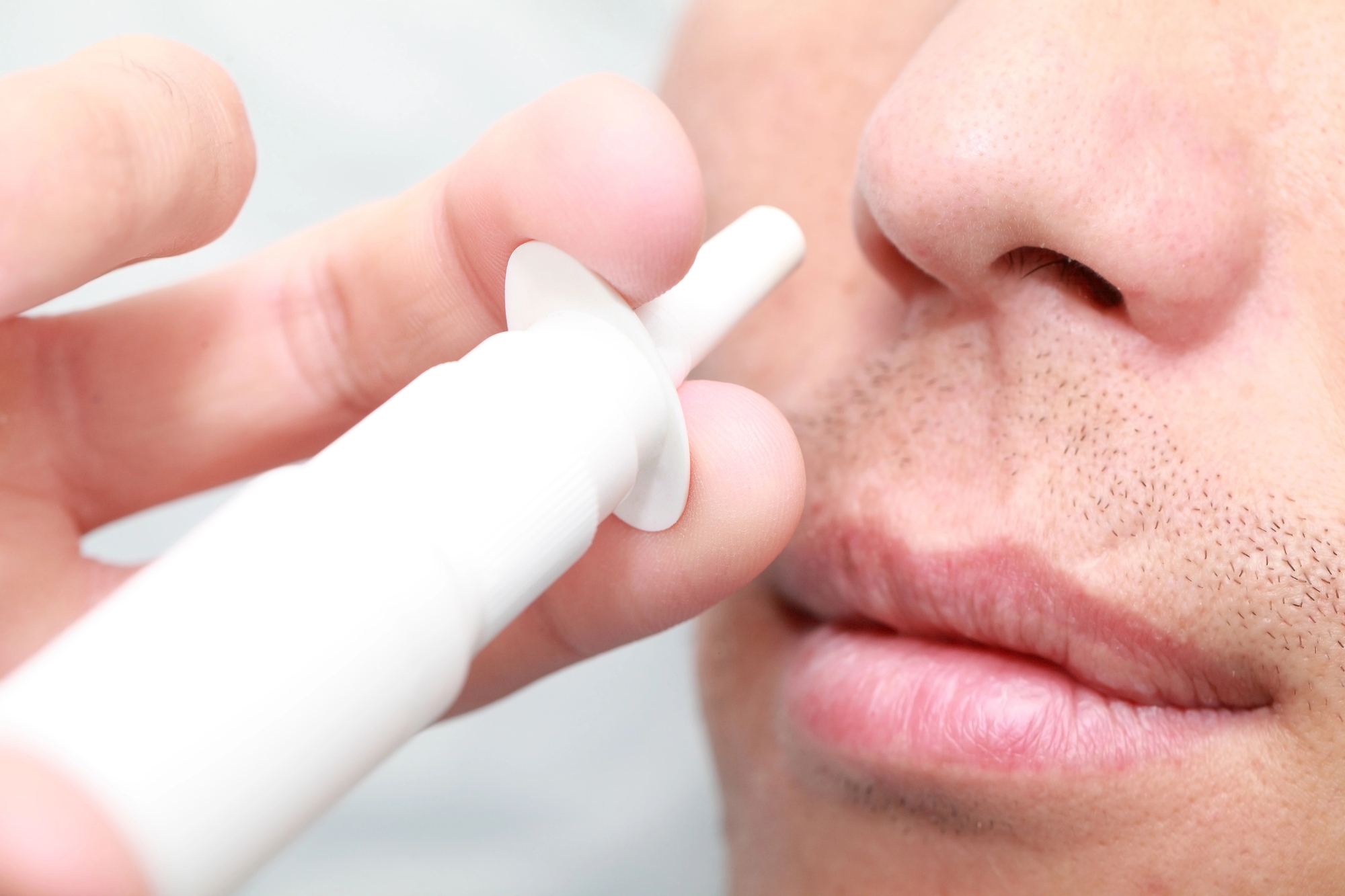
An innovative, noninvasive Israeli medication administered through the nose hopes to be a life-changer for people suffering acute spinal cord injuries – even helping them to walk again.
NurExone Biologic Inc. developed ExoPTEN, which treats damage to the nervous system, from a concept created by two members of its Scientific Advisory Board: Prof. Shulamit Levenberg of the Technion – Israel Institute of Technology and Prof. Daniel Offen from Tel Aviv University.
(Another member of the board is Prof. Gabriel Zeilig, the head of the National Spinal Cord Injury Rehabilitation Unit at Sheba Medical Center, Israel’s largest and globally renowned hospital.)
According to the World Health Organization, up to half a million people globally sustain a spinal cord injury every year, most from preventable causes such as road accidents or falls.
Furthermore, the WHO says, people suffering from spinal injuries are up to five times more likely to die prematurely and are less likely to complete their education or find economic stability.
The NurExone concept is two-fold: the medication’s active ingredient and its delivery via the nose.
The two scientists spent many years working together on regeneration in spinal cord injuries using stem cells. Their breakthrough came, however, when they started using exosomes – nanoscopic particles released by cells to pass messages to one another, over both short and long distances.
Crucially, these exosomes can penetrate through cell membranes and even the brain-blood barrier (BBB), a border made of cells that prevents almost all substances from entering or leaving the brain.
Exosomes, NurExone CEO Lior Shaltiel tells NoCamels, are like mirror images of the cells that secrete them. So whether they are taken from bone marrow or umbilical cord (stem) cells, exosomes contain the same protein, RNA or DNA as their creators.
NurExone decided that exosomes taken from bone marrow cells had the most potential to help regenerate cells affected by a spinal cord injury or traumatic brain injury.
“The bone marrow – in our case – looked to be the best and the most potent exosomes,” Shaltiel explains.
From there, the company added the active ingredient – a form of RNA known as siRNA, which prevents restrictions on the regeneration of cells, a key aspect of healing spinal cord injuries.
The exosomes laden with the siRNA enter through the nose and are delivered to the injured areas of the spine, having passed through the brain blood barrier.
These exosomes then communicate to healthy cells in the area to flock to the injured region in order to aid regeneration.
“They want to help and assist damaged cells in damaged tissue,” Shaltiel explains.
When the exosomes reach the damaged cells, they enter and dissolve, releasing the siRNA that then blocks the protein hampering regeneration at the injury site.
One of the advantages of exosomes, Shaltiel says, is that they are suitable for everyone, with no adverse reactions being recorded in any individuals – which would require tailoring the therapy to different needs.
“They’re very safe. You don’t see side effects, they have no immune response,” he explains. “This is a huge advantage operation-wise when trying to develop a new drug.”
When the treatment is underway, changes to the damaged area can be observed via MRI scans, which will display any regeneration and repair of the injured tissue.
The therapy has successfully been trialed in lab rats with acute spinal cord injury. The rats were administered the medication through the nose immediately after their injury for five consecutive days, followed by four days of rest, and then another five days of treatment.
According to NurExone, 75 percent of the test cases permanently recovered at least some motor function, including being able to walk.
“It’s there to stay,” Shaltiel says of the recovery. “It’s not going to become worse.”
In October 2023, NurExone received orphan drug status (therapies that show promise in the prevention, diagnosis or treatment of rare diseases) from the US Food and Drug Administration.
And despite still being far from ready for market, NurExone is already a publicly traded company, with shares on Canadian and German stock exchanges, alongside private investors.
The Technion is one of those shareholders and earlier this month the company announced it was moving into a new state-of-the-art R&D facility on the campus.
Shaltiel hopes to enter clinical trials for humans in the near future and the company is currently working on approval for this in North American hospitals, where the number of candidates is far larger than it is in Israel.
He also hopes to enter into a “significant partnership” with a large biopharmaceutical company that would be interested in expanding into the field of exosomes as a treatment.
“In the end,” he says, “we are the pioneers in this field.”